Our perspectives
in one place
Topic
Selected filters
ClearNews
See allAMATA statement at 75th WHO AFRO: Progress report on the regional strategy on regulation of medical products in African region
On 27 August 2025, the African Medicines Agency Treaty Alliance submitted a statement on agenda item 16.8: Progress report on the regional strategy on regulation of medical products in African region, 2016-2025 at the 75th Session of the WHO Regional Committee for Africa. The African Medicines Agency Treaty Alliance (AMATA) commends the progress made under...
Read more75th WHO Regional Committee for Africa: Progress report on the implementation of the strategy for scaling up health innovations in the African region
On 27 August 2025 in Lusaka, Zambia, IFPMA delivered a statement at the 75th session of the WHO Regional Committee for Africa focused on Agenda item 16.2: Progress report on the implementation of the strategy for scaling up health innovations in the African region. IFPMA takes note of WHO AFRO’s Progress report on the implementation...
Read moreAdvancing a health-centric global plastics treaty
On 5 August 2025, IFPMA, together with Health Care Without Harm and the Global Self-Care Federation, published a joint statement on the global plastics treaty under negotation at the continued fifth session of the Intergovernmental Negotiating Committee (INC-5.2) in Geneva.
Read moreHigh-level interactive dialogue on the social, economic, and environmental determinants of health
On 11 July 2025 in New York at the UN Headquarters, IFPMA delivered a statement at the High-Level Dialogue on the Determinants of Health. Innovation in public health is essential to addressing the social, economic, and environmental determinants of health. IFPMA welcomes this dialogue and underscores the importance of collaboration to address complex health challenges,...
Read more
Groups representing patients, healthcare professionals and pharmaceutical industry author new principle on use of AI in healthcare
Read moreThe pharmaceutical industry supports Gavi’s high-level pledging meeting
On 25 June 2025, Gavi, the Vaccine Alliance, is co-hosting the Global Summit: Health & Prosperity through Immunization in support of its next five-year strategic period, in partnership with the European Union and the Gates Foundation.
Read morePublications
See allReimagining our response to non-communicable diseases (NCDs) and mental health
Reflections on the draft Political Declaration for the UN High Level Meeting on the Prevention and Control of NCDs and the Promotion of Mental Health and Well-being This advocacy brief outlines three areas where industry seeks more explicit commitments from governments: Leverage health innovation Strengthen prevention and early action for lifelong health Address multimorbidity through...
Read more
International consensus framework for ethical collaboration in health
The International Consensus Framework for Ethical Collaboration in Health is the only global platform of its kind, routinely convening diverse health stakeholders in support of high-quality patient care. First established in 2014, the Consensus Framework was revised in 2024 to coincide with its tenth anniversary and adopted in 2025 to include a new principle, on responsible use of health data and technology. This reflects a growing importance of digital health and artificial intelligence, and a need for ethics to evolve alongside innovation.
Read more
Key access pathways and bottlenecks for medicines in LMICs
Medicines and vaccines have transformed the way we prevent, treat, and cure diseases. Bringing these to people around the world requires more than scientific breakthroughs – it demands collaboration across the entire innovation and access pathway. From discovery and development to regulatory approval, supply, procurement and local delivery, each step plays a critical role in...
Read more
Expert insights
See all
Getting procurement right: sustainable and secure supply chains that support access to medicines
Read more
G7 Canada is an opportunity to unlock the potential of life sciences
Read more
Global resilience and national security starts with preventing ill health
Read moreResources
See allStreamlining samples management to strengthen health outcomes in Africa
Ensuring the quality, safety, and efficacy of pharmaceutical products is a cornerstone of public health. To meet these high standards, innovative multinational pharmaceutical companies perform rigorous batch release testing for every product, following internationally recognized protocols and approved specifications. However, countries may require additional in-country testing by their National Regulatory Authorities (NRAs) or National Control...
Read more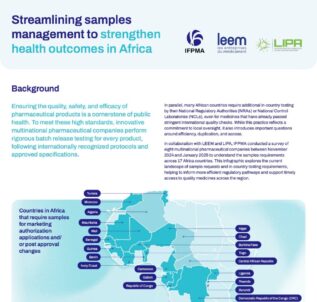
Our Ethos in Action – Decision-Making Framework Toolkit
IFPMA has developed a Five-Phase Decision-Making Framework, grounded in the IFPMA Ethos or value system, to help companies make decisions that balance business objectives and ethical considerations to meet patient needs and the expectations of the medical community, regulators, and society.
Read more
February 2024: Impact of a waiver of intellectual property rights for COVID-19 therapeutics
As discussions on an extension of a waiver of intellectual property (IP) rights on COVID-19 therapeutics continue, latest evidence and data published today explains what the adverse impact of a waiver may be on the entire innovation ecosystem and the consequences it may have on industry’s ability to fight future pandemics.
Read more


